Batters And Doughs. Part 2
Description
This section is from the book "Experimental Cookery From The Chemical And Physical Standpoint", by Belle Lowe. Also available from Amazon: Experimental cookery.
Batters And Doughs. Part 2
Many old recipes containing molasses, like those containing sour milk, call for excess soda. In the cooky contest previously mentioned, it was not uncommon to find recipes that called for 1/2 cup of soda for 3 quarts of molasses and a pint of sour cream or milk.
Honey and corn sirup. The acidity of honey as reported by Daniels and Heisig varies. For the samples they tried they report that from 1/2 to 1/12 of a teaspoon of soda was required to neutralize the acidity of 1 cup of honey. This acidity is so low that it is hardly worth while to add soda for neutralizing. The acidity of corn sirup is very much lower than that of honey.
Cream of tartar. Cream of tartar is an acid salt and combines with soda to form carbon dioxide gas. It was used extensively before baking powders came into common use. Many recipes still state to use cream of tartar and soda for the leavening agent, and many of them call for equal amounts of these two ingredients. As the weight of a teaspoon of soda or of cream of tartar is practically the same, an excess of soda is left in the baking mixture when equal measures of the two are used, for by weight 188 parts of cream of tartar combine with 84 parts of soda to form 18 parts of water, 44 parts of carbon dioxide, and 210 parts of sodium potas-sium tartrate. These proportions by measure are approximately 2 1/4 tea-spoons of cream of tartar to a teaspoon of soda.
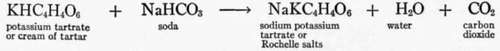
Baking Powder
Hart states that baking powder was first used in England about 1830. It was made of "pearlash and alum, and later of sesquicarbonate of ammonia." When crystalline substances were mixed with soda, a wider use was insured for baking powder, for with the dry salts, reactions of the baking powder did not take place until it was moistened, and the alkaline and acid substances could be kept in the same container.
The ideal baking powder. Hart states that "From the standpoint of the consumer, the ideal baking powder (a) gives the most gas for the least volume and weight of powder; (b) gives the gas slowly when cold and increasingly in the cooking dough, so the dough may be mixed cold and left standing several hours; begins to generate gas in quantity in the oven and ceases to generate when it would rupture the crumb; (c) leaves a tasteless and absolutely harmless residue in the bread; (d) is cheap; (e) keeps well. The chemicals should not react on one another in the can and thus lose strength."
Carbon dioxide. Under the United States Food and Drugs Act, the following definition for baking powder was formulated. "Baking powder is the leavening agent produced by the mixing of an acid reacting material and sodium bicarbonate with or without starch or flour.
"It yields not less than 12 per cent of available carbon dioxide."
The principal reason for adding starch to baking powder is for a standardizing agent. To some extent it also absorbs moisture, thus lessening the gas lost during storage by reaction of the ingredients and for a standardizing agent. The federal standard requires 12 per cent of available carbon dioxide, but most of the baking powders made for home use yield 14 per cent of carbon dioxide. Starch is added to each type of baking powder in amounts so that 100 grams of the powder yield 14 per cent carbon dioxide. If the amount of gas produced by a definite weight of baking powder were not standardized there would be great difficulty in substituting some types of powders for others in recipes. For under these conditions the amount of carbon dioxide produced by a teaspoon of powder would vary far more than it does when the powder yields 14 per cent of carbon dioxide.
The carbon dioxide of baking powders is classified as total, available, and residual. The total carbon dioxide is all the carbon dioxide contained in the powder and may be obtained by using acid with it. The residual carbon dioxide is that remaining in the powder after water has been mixed with it and heat has been applied. Available carbon dioxide represents the difference between total and residual and is the amount usually obtained under baking conditions.
Types of baking powders. The alkaline ingredient of all baking powders is soda. The acid ingredient varies, and the names of the various types of baking powders refer to the kind of compound used for the acid ingredient. The function of the acid salt is the same regardless of the type of salt used. It combines with and releases the CO2 from the soda. The types of baking powders are (1) tartrate, (2) phosphate, (3) sulfate, and (4) a combination of sulfate and phosphate.
Tartrate powders. The tartrate powders on the market consist of cream of tartar or a combination of cream of tartar and tartaric acid. Tartaric acid is not used alone in baking powders. The objections to using it alone as given by Hart are that "Its reaction is rapid and complete in cold water, and its keeping quality correspondingly poor." Cream of tartar reacts more slowly than tartaric acid but keeps and aerates well.
The residue left from cream of tartar and soda is "Rochelle" salts. See the reaction under cream of tartar. Tartaric acid leaves sodium tartrate as a residue.
Phosphate powders. Three phosphate compounds are or have been used in baking powder. Monosodium phosphate has been used, but so far as the author knows no baking powders on the market at present contain it. Monocalcium phosphate is most frequently used. Lately sodium acid pyrophosphate is being used in considerable quantities by commercial bakers. Phosphoric acid forms three series of salts, such as Na3PO4, Na2HPO4, and NaH1PO4. The first is not formed in baked products. Davis and Maveety state that the monosodium phosphate (NaH1PO4), disodium phosphate (Na2HPO4), tricalcium phosphate (Ca3(PO4)2), and dical-cium phosphate (CaHPO4) are found in the residue after the baking powder has reacted. They state that the reaction is carried to completion with the formation of Na2HPO4 and Ca3(PO4)2 only when an excess of hydroxyl ions is present.
Continue to:


