The Approximate Percentage Of Unsaturated Glycerides Found In Some Fats And Oils. Continued
Description
This section is from the book "Experimental Cookery From The Chemical And Physical Standpoint", by Belle Lowe. Also available from Amazon: Experimental cookery.
The Approximate Percentage Of Unsaturated Glycerides Found In Some Fats And Oils. Continued
Texture and consistency of fats. The texture or consistency of a fat, and hence its properties in baked products, is not only influenced by its component glycerides, but also by the rate of cooling and mechanical agitation. For example, if a sample of lard is divided into two parts, and one portion is cooled slowly, i.e., kept in a warm room or an incubator just below the congealing point of the fat, the glycerides with high melting points tend to crystallize and separate from those with low melting points. As a result the crystallized glycerides form granular spots surrounded by oil. If the second portion of lard is chilled rapidly, there are fewer granules and the fat is not so grainy. If in addition to rapid chilling, the fat is also stirred while it is being cooled it becomes very smooth. If the temperature to which it is cooled is several degrees below its melting point the fat may become waxy and so firm that it is rather difficult to cream at ordinary temperatures. The oil does not separate from the smooth fat as the fat becomes soft. It is for this reason that only smooth lard or fat is sold in paper cartons. Grainy lard may be purchased but comes in metal containers. The commercial smooth lards are not cooled sufficiently to become waxy.
Fatty acids commonly found in foods. The fatty acids commonly found combined with the glycerol in food and cooking fats are palmitic, stearic, and oleic. Chemically the fatty acids of the acetic acid series have the general formula CnH1nO2.. The most important of this series in foods are the palmitic and stearic, though butyric and caproic, with small amounts of caprylic and capric, form about 7 per cent of the glycerides of butter.
Butyric acid

Caproic acid
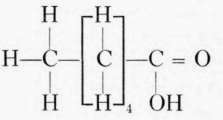
Caprylic acid
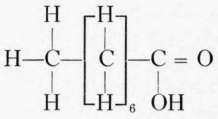
Capric acid
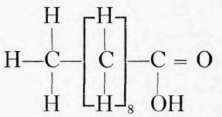
Palmitic acid
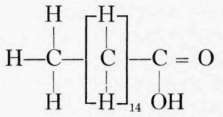
Stearic acid
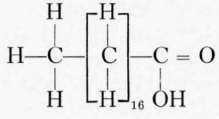
Of the fatty acid series with the general formula CNH1NO oleic acid is the most important in food fats. The acids of this series contain one double bond in the chain and are unsaturated.
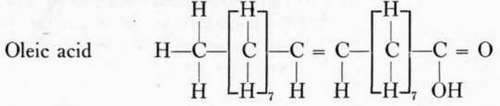
Linolic acid belongs to the fatty acid series with the general formula CnH1n-402. Linolic and linolenic acids are very important because they are necessary for proper functioning of the body, and the animal body does not have the ability to synthesize the unsaturated fatty acids. Linolic acid contains two double bonds.
Linolenic acid belongs to the series CnH1n-6O2 and contains three double bonds.
When oils or fats are hydrogenated, it is generally agreed that the more highly unsaturated acids are the first to become saturated.
Though present in only small amounts both linolic and linolenic acids may influence the properties of foods. Their effect, or the effect of their oxidation products, on gluten quality has been considered. The glycerides with more than one double bond also oxidize readily. The shortening power of a fat or oil because of the number of double bonds it contains is still to be discussed.
Glycerol. Glycerol or glycerin and fatty acids are formed by hydrolysis of the fats by enzymes during digestion or by saponification, i.e., hydrolysis by alkalies. Glycerine has the ability to retain moisture to a high degree, being more hygroscopic than the levulose compounds. It has long been used by many European bakers to retain the moisture in cakes, cookies, etc.
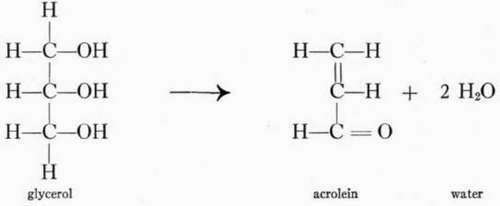
Heat decomposition products of glycerol. Some free and uncom-bined fatty acids and glycerol are usually found with the fats. When heated to a high enough temperature the glycerol decomposes, losing two molecules of water and forming acrolein. Acrolein is an aldehyde that has a sharp odor and is irritating to mucous membranes of the nose and throat and to the eyes. The odor and irritation are familiar to all who have had experience with "burning fats."
Free fatty acids. The amount of free fatty acids found in some fats and oils is greater than in others. As previously stated, upon hydrolysis, which may be brought about by enzymes, the lipases, or by boiling with alkali, fats and oils split into glycerol and fatty acids. In cooking materials in fat, particularly foods with considerable water content, the percentage of free fatty acids is increased. During heating in the presence of moisture some hydrolysis occurs, yielding glycerol and free fatty acids.
Continue to:


