Ionone
Description
This section is from the book "The Volatile Oils Vol1", by E. Gildemeister. Also available from Amazon: The Volatile Oils.
Ionone
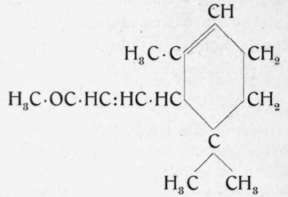
A-Ionone.

B-Ionone
This violet perfume of the formula C13H20O, the presence ) which in nature has not yet been definitely established, was first obtained synthetically in 1893 by Tiemann and Kruger.1) Since then a considerable number of patents have been issued for the preparation of "violet ketones" /'. e., for that of ionone and its homologues.
1) Berl. Berichte 33 (1900), 275, 2454.
2) Liebig's Annalen 323 (1902), 371.
3) Ibidem 275 (1893), 179; 286 (1895), 109.
The preparation of ionone is based on the condensation of the olefinic aldehyde citral with acetone. In the presence of alkalies the olefinic ketone, the pseudoionone of the formula C13H20O, results. In the presence of acid reagents, whether weak or strong, either at low or higher temperatures, this pseudoionone rearranges itself to the cyclic, isomeric ketone, the ionone.
>C:CH.CH2.CH2.C:CH.CH0H-CH3C0CH3 = H20 + ch/ I
Citral. CH3 Acetone.
CH3x
/ C: CH • CH2 • CH2 • C: CH • CH: CH • CO • CH3 -> Ionone.
CH/ " I
CH3
Continue to:


