General Remarks. Part 11
Description
This section is from the book "Distillation Principles And Processes", by Sydney Young. Also available from Amazon: Distillation Principles And Processes.
General Remarks. Part 11
Ethyl Alcohol - Benzene - Water
The behaviour of mixtures of ethyl alcohol, benzene, and water has already been referred to (p. 179); but it may be pointed out that, when a mixture of equal weights of benzene and of 95 per cent alcohol is distilled through a very efficient still-head, the substance of highest boiling point, water, comes over in the first of the three fractions into which the distillate tends to separate, the remainder of the benzene in the second fraction, while the third fraction or residue consists of the most volatile of the original components, alcohol.
Aliphatic Acids And Water
Again, when a mixture of formic, acetic and butyric acids with water is distilled, it tends to separate into three or more of the following components.
Boiling point. | |
1. Butyric acid-water (mixture of minimum boiling point) | 99.4° |
2. Water........... | 100 0 |
3. Formic acid......... | 100.7 |
4. Formic acid-water (mixture of maximum boiling point) | 107.3 |
5. Acetic acid . . . . . . . . . . . . . . . . . | 118.5 |
6. Butyric acid......... | . 163.5 |
With a large amount of water, the whole of the butyric acid would come over in the lowest fraction, and if the amount of acetic acid was large, the last fraction would consist of that acid ; but although acetic acid does not form a mixture of minimum boiling point with water, yet it is very difficult to separate the acid from its dilute aqueous solution by distillation, and if the amount of the acid was relatively small it might all be carried over with the water at temperatures below 107°, and in that case the highest fraction would consist of the formic acid-water mixture of maximum boiling point. The acids would then come over in the reversed order of their boiling points. Hecht1 found that on distilling a mixture of acetic, butyric, and oenanthylic acids with much water, the whole of the oenanthylic acid came over in the first portion of the distillate, the middle portion contained chiefly butyric acid and the last portion contained acetic acid nearly free from the other two. Hecht points out that acetic acid is miscible with water in all proportions with considerable heat evolution ; butyric acid is also miscible with water in all proportions but very little heat change is observable, and oenanthylic acid is nearly insoluble in water.
The determination of the qualitative and quantitative composition of mixtures of fatty acids is a difficult problem. The methods suggested for the estimation of the acids are based either (1) on the relative solubilities of the acids or of their salts, or (2) on the relative rates of distillation of the acids or of their esters. The methods depending on the rates of distillation of the acids volatile with steam have been examined and are discussed by Reilly,2 and he has devised a process by which a single acid in dilute aqueous solution may be identified or the percentage amounts of two or more acids in dilute solution may be determined.
1 Hecht, "On Isoheptoic Acid from /3-Hexyl Iodide," Liebig's Annalen, 1881, 209., 321.
2 Reilly, " The Determination of the Volatile Fatty Acids by an Improved Distillation Method," Sci. Proc. Boy. Dubl. Soc., 1919, 15 (N.S.), 513.
Butter Fat
Butter fat is a complex mixture of the glycerides of a considerable number of fatty acids. The composition is, no doubt, somewhat variable, but the table below, due to Brown, will at any rate give an idea of the nature and the relative quantity of these glycerides.
Table 81
Glycerides of | Per cent | |
Dihydroxy stearic acid . | C18H34(OH)2O2 | 104 |
Oleic acid...... | C18H34O2 | 33.95 |
Stearic acid...... | C18H3O2 | 1.91 |
Palmitic acid...... | C16H32O2 | 40.51 |
Myristic acid . . . . , . | C14H28O2 | 10.44 |
Laurie acid...... | C12H24O2 | 2.73 |
Capric acid...... | Cl0H20O2 | 0.34 |
Caprylic acid...... | C8H1602 | 0.53 |
Caproic acid...... | C6H12O2 | 2.32 |
C4H8O2 | 6.32 |
In the analysis of butter fat, when both the soluble (Reichert-Wollny-Meissl method) and insoluble (Polenske method) volatile fatty acids are to be estimated, 5 grams of the fat, 20 grams of glycerol and 2 c.c. of 50 per cent aqueous (not alcoholic) caustic soda are heated in a 300 c.c. flask until saponification is complete. The soap is dissolved in 90 c.c. of hot water; 50 c.c. of normal sulphuric acid and about 0.1 gram of finely powdered pumice are added, and the mixture is distilled at such a rate that 110 c.c. come over in about 20 minutes. The receiver (flask) is then replaced by a 25 c.c. cylinder and the distillation is stopped. The well cooled distillate in the flask, after standing, is filtered and 100 c.c. of the filtrate is used for the estimation of the soluble volatile acids. The insoluble volatile acids are carefully collected from the flask, cylinder, and condenser, dissolved in alcohol and separately estimated.
Of the fatty acids present in butter, butyric acid is the only one which is miscible with water in all proportions. It forms with water an azeotropic mixture which, according to Lecat, boils at 994° under normal pressure and contains 18.4 per cent of the acid.
It may therefore be fairly assumed that the whole of the butyric acid from the butter fat is contained in the 110 c.c. of distillate.
As regards the higher acids, as the molecular weight increases the solubility in water diminishes, palmitic, stearic, and oleic acids, at any rate, being practically insoluble. All these higher acids form, of course, azeotropic "mixtures" - termed by Lecat "heterogeneous mixtures" - with water, but the vapour pressures of palmitic, stearic, and oleic and dihydroxy stearic acids are so low that the quantities that distil over with steam must be exceedingly small.
V. H. Kirkham,1 Government Analyst, East Africa Protectorate, whose laboratory at Nairobi is 5,500 ft. above sea level, having invariably obtained very low values by the Polenske method in the analysis of butter, was led to investigate the influence of pressure on the Reichert-Meissl and Polenske values, and found that as the pressure was increased the Reichert-Meissl value rose slowly while the Polenske value rose very rapidly.
1 V. H. Kirkham, "The Effect of Pressure upon the Polenske and Reichert-Meissl Values," The Analyst, 1920, 45, 293.
Kirkham made determinations of both the soluble and insoluble volatile fatty acids at pressures from 100 to 1000 mm. and found that the Polenske values were satisfactorily reproduced by the formula where V and v are the Polenske values corresponding respectively to the pressures P and p, and the constant k = 45.
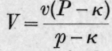
The Reichert-Meissl values agreed moderately well with the formula
The observed and calculated values are given below.
Continue to:


