4. Oxidations
Description
This section is from the book "The Fundamental Processes Of Dye Chemistry", by Dr. Hans Eduard Fierz-David. Also available from Amazon: The Fundamental Processes of Dye Chemistry.
4. Oxidations
Dinitrostilbene-Disulphonic Acid and Diaminostilbene-Disulphonic Acid from p-Nitrotoluene.
(Conjoint oxidation of two molecules.) Reaction:
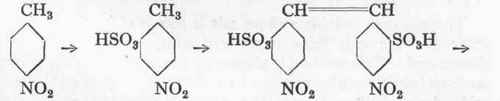
1 Cf. also Malachite Green and Xylene Blue VS.
(a) Dinitrostilbene-Disulphonic Acid.
oo Gms. p-nitrotoluene are sulphonated exactly as described for nitrobenzene, and the product separated as the sodium salt. The press-cakes are dissolved in 500 c.cs. of water at 6o° with the aid of soda, about 50 gms. being required; if more be needed, the cakes were insufficiently pressed. The solution is filtered from iron oxide which is nearly always present, and made up to 2 litres at 500. To the well-stirred liquid 160 gms. of 35 % caustic soda lye are added during half an hour; no sodium salt of the sulphonic acid should separate out. A mixture of 1700 gms. sodium hypochlorite solution containing about 5 % NaOCl, and 300 gms. of 35 % caustic soda lye is then allowed to drop in similarly during 10 hours. The strength of the hypochlorite must be exactly determined by titration. It should be remembered that only hypochlorite solutions containing at least 5 % excess NaOH will keep, which is of special importance as regards the preparation of the hypochlorite solution. The temperature must not exceed 560, as otherwise yellow dyes of the Mikado series are formed.
The mixture is now allowed to stand at 550 for at least 24 hours, taking care that free chlorine (hypochlorite) can be detected during the whole period with the aid of potassium iodide-starch paper. It is then cooled to 15o, 400 gms. of salt are added, and the whole is allowed to stand for a day. The sodium salt of dinitrostilbene-disulphonic acid separates out as a yellow crystalline precipitate which is filtered and washed with a very little brine. The yield of crude salt is about 100 gms.
100 gms. p-Nitro toluene.
280-320 gms.
25 % Oleum.
300 gms.
H2O.
300 gms. Ice.
250 gms.
NaCl.
50 gms.
Soda.
2 1. Water.
160 gms.
35 % NaOH.
1700 gms.
5% NaOCl
(=85 gms. 100 % NaOCl). 300 gms. NaOH (35 %)•
400 gms. NaCl.
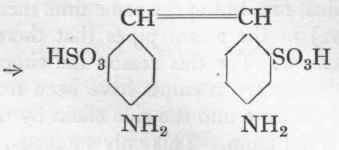
(b) Reduction to Diaminostilbene-Disulphonic Acid.
The sparingly soluble sodium salt is dissolved in 300 c.cs. hot water, the free soda being neutralized with a little dilute hydrochloric acid. This solution is allowed to run on to 200 gms. of iron turnings (which have been etched by means of 20 c.cs. of 40 % acetic acid), during half an hour. The reduction proceeds according to the known method (see, for example, p. 67).
About
300 c.cs.
water.
A little Hc1.
200 gms. Fe.
20 c.cs.
40% Acetic acid.
The clear solution is made strongly acid to Congo with hydrochloric acid, whereupon the diaminostilbene-disulphonic acid is precipitated in small yellowish-white crystals. It is filtered off after standing for 10 hours and thoroughly washed. The yield of sulphonic acid for each 100 gms. p-nitrotoluene is about 75 gms. of 100 % product. In distinction from the analogously constituted 2:2'-benzidine disulphonic acid, it cannot easily be diazotized by the indirect method.
Notes on Works Technique and Practice. - The method for the preparation of diaminostilbene-disulphonic acid described here was first given by Green, and, with the lowering of the price of chlorine, it has completely displaced Leonhardt's method. The old process consisted in reducing Mikado Yellow, which was obtained by the action of concentrated soda lye upon p-nitrotoluene sulphonic acid. By this method, however, only 48 % of the theoretical yield of diamino acid is obtained even under the most favourable conditions, and large quantities of zinc dust or ammonium sulphide are required for the reduction. Further, all the caustic soda is lost, whilst by Green's process caustic lye, chlorate and common salt can be recovered. Again Green's product is much purer; if too concentrated solutions have not been used it contains absolutely no diamino-dibenzyl disulphonic acid, which weakens Chrysophenin considerably. The presence of the dibenzyl derivative can be readily detected by means of two reactions: first, the dye from H-acid and the dibenzyl acid is much redder than that from the stilbene derivative, and secondly, the "Chrysophenin " from the dibenzyl derivative turns almost reddish-violet with mineral acids, and not a pure blue. A comparatively small content of diamino-dibenzyl disulphonic acid can be recognized at once by comparison with a specimen of the pure colour. - The oxidation to the dinitro acid is carried out in concrete vats. It is to be noted that a very small content of iron or even of copper immediately decomposes the hypochlorite solution, wood being inadmissible also for the same reason.
About 15 gms. Na2Co3. About 100 gms.
30% Hc1.
Continue to:


