Behavior On Boiling And Fractional Distillation
Description
This section is from the book "The Volatile Oils Vol1", by E. Gildemeister. Also available from Amazon: The Volatile Oils.
Behavior On Boiling And Fractional Distillation
Inasmuch as the volatile oils are mixtures of substances with different boiling points, it is improper to speak, as is so often done, of the boiling point of a volatile oil. It is more correct to speak of a boiling temperature, by which is meant the temperature interval within the limits of which the oil distills over in a single distillation from an ordinary distilling flask (fig.70) without the application of a fractionating arrangement. It is of the greatest importance that the entire column of the mercury be surrounded by the vapors of the distilling oil, a circumstance that is often ignored and leads to erroneous results. The thermometer should be adjusted in such a manner that the respective boiling point is placed slightly above the exit tube of the distilling flask. Care, however, should be exercised that the mercury bulb of the thermometer does not project into the body of the flask, much less into the liquid. In order to avoid this, thermometers with shortened scales are preferably used. Of great importance is also the rate of distillation which is best regulated so that 40 or at most 60 drops are collected per minute. If the distillation is conducted too rapidly and if the exit tube is not large enough the vapors are choked causing an increase in pressure and a corresponding rise in temperature. The consequence is this that the observed boiling point is too high. In order to avoid bumping, small pieces of pumice, tiling or or talcum are added to the liquid.

Fig. 70.
Whereas the determination of the boiling point is of importance in the examination of chemical individuals, fractional distillation yields better results in the examination of volatile oils. The several fractions are collected separately, their amounts ascertained, and each fraction examined separately if necessary. The observations recorded by different observers of the amounts of the same oil which distill over between certain limits of temperature, seldom agree, because the results are not only greatly influenced by the form of the distilling flask, but also by the rapidity of the distillation and the height of the barometer. In the examination of certain fractions of individual oils it is therefore necessary, to use flasks of fixed dimensions and to observe a certain rapidity of distillation. For testing lemon oil, rosemary oil and spike oil, Schimmel & Co. use distilling flasks according to Ladenburg of the dimensions given in fig. 71.1) From 50 cc.
of the oils mentioned, 5 cc. are distilled over in such a manner that about 1 drop falls in a second, and the distillate tested in the polariscope, as will be described more in detail under the individual oils.
When the different constituents are to be isolated from an oil, the fractional distillation must be repeated many times and preferably by employing one of the well known distilling columns. In order to avoid decomposition, it is best to distill the fractions boiling above 200° in vacuum. Oils containing esters must first be saponified, as the acids, which are easily split off by the boiling, disturb the fractionation and may act upon the other constituents of the oils.
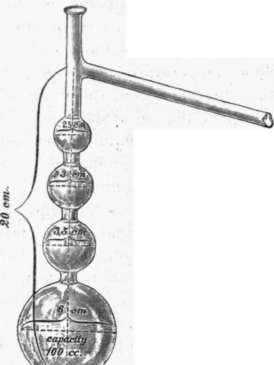
Fig. 71.
Continue to:


