"Simple" And "Mixed" Triglycerides
Description
This section is from the book "Chemistry Of Food And Nutrition", by Henry C. Sherman. Also available from Amazon: Chemistry of food and nutrition.
"Simple" And "Mixed" Triglycerides
Triglycerides in which the three fatty acid radicles are of the same kind are known as simple triglycerides. Tristearin, triolein, tripalmitin, etc., are examples of simple triglycerides. A mixed triglyceride is one in which the three fatty acid radicles are not all of the same kind. For example, distearo-olein (having two radicles of stearic and one of oleic acid), dioleo-palmitin (having two of oleic and one of palmitic), or stearo-oleo-palmitin (having one radicle each of stearic, oleic, and palmitic acids), is each a mixed triglyceride.
It is evident from the chemical structure of glycerol that there can be only one simple triglyceride of any given fatty acid but that with two fatty acid radicles alike (R2) and one different (R1) the triglyceride may have either of the following forms:
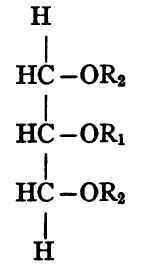
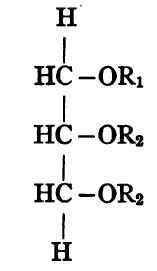
That is, the two radicles of the same kind may be on the terminal carbons or may be adjacent. It will be noted that the two substances here represented have exactly the same composition, but different constitution.
If now the triglyceride contains one each of three different acid radicles (R1, R2, R3) there are plainly three possible forms:
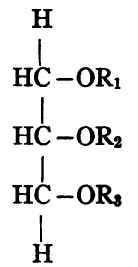
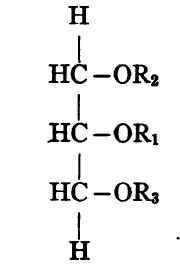
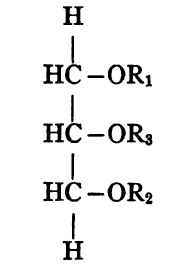
Each of these three substances has exactly the same composition, though the constitution is different for each.
It should be noted that these five formulae represent types of structure and that the actual number of triglycerides possible from three fatty acid radicles is greater since we may have substances corresponding to either of the first two in which R3 replaces either R1 or R2; and it is plain that as the number of fatty acids is increased beyond three the number of possible mixed triglycerides increases very rapidly so that with the large number of fatty acids which are now known to be of fairly common occurrence in fats the possible number of mixed triglycerides must be almost unlimited. The simple triglycerides corresponding to the common fatty acids are all known, but naturally not all of the practically innumerable possible mixed triglycerides have been separated or prepared.
Berthelot in 1869 suggested that fats probably contain mixed glycerides and in 1889 Blyth and Robertson reported a palmito-stearo-olein in butter, but it is only since Kreis and Hafner (1903) described the preparation of palmito-distearin from beef tallow and Bomer (1909) separated stearo-dipalmitin from mutton tallow and palmito-distearin from lard that the widespread occurrence of mixed glycerides in the familiar fats has been generally accepted.
Among the other mixed glycerides reported as having been isolated from natural fats are:
Myristo-palmito-olein in cacao butter (Klimont, 1902), dipalmito-olein and stearo-palmito-olein in tallow (Hansen, 1902), distearo-olein in cacao butter (Fritzweiler, 1903) and in Borneo tallow (Klimont, 1905), stearo-diolein in human fat (Partheil and Ferie, 1903).
The fact long known to analysts that fats too nearly identical in composition to be distinguished by chemical analysis may still show differences in crystalline structure under the microscope is now explained as due to the presence of different mixed triglycerides containing the same fatty acid radicles. Thus beef fat rendered at such a temperature as to contain the glycerides of stearic, palmitic, and oleic acids in practically the same proportions as in lard still differs so constantly from lard in its microscopic appearance as to indicate the presence of distinct chemical substances and has now been shown to contain different mixed triglycerides.
The fact that tributyrin has an intensely bitter taste makes it seem probable that none of this substance occurs in butter but rather that the butyric acid in butter fat is in the form of mixed glycerides. Probably mixed glycerides are as abundant as simple glycerides in natural fats.
Formation And Composition Of Natural Fats
Fats are formed both in plants and in animals. The conditions which determine fat formation, and the character of the fat formed in different species and under different conditions, are better known than the chemical steps involved in the process. It is hardly necessary to mention the fact that the true fats are composed of the same three chemical elements of which the carbohydrates are composed (carbon, hydrogen, and oxygen) and that since the fats contain less oxygen and more carbon and hydrogen than the carbohydrates, they constitute a more concentrated form of fuel or a more compact and lighter medium for the storage of energy for future use. The question therefore presents itself whether either the plant or the animal organism (or both) has the power to change carbohydrate material into fat.
Continue to:


