Vincent's Chloride Of Methyl Ice Machine
Description
This section is from "Scientific American Supplement Volumes 275, 286, 288, 299, 303, 312, 315, 324, 344 and 358". Also available from Amazon: Scientific American Reference Book.
Vincent's Chloride Of Methyl Ice Machine
Chloride of methyl was discovered in 1840 by Messrs. Dumas and Peligot, who obtained it by treating methylic alcohol with a mixture of sea salt and sulphuric acid. It is a gaseous product at ordinary temperature, but when compressed and cooled, easily liquefies and produces a colorless, neutral liquid which enters into ebullition at 237.7° above zero and under a pressure of 0.76 m.

VINCENTS ICE MACHINE. FIG. 1.--THE FREEZER (Longitudinal Section).
Up to recent times, chloride of methyl in a free state had received scarcely any industrial application, by reason of the difficulty of preparing it in a state of purity at a low price. Mr. C. Vincent, however, has made known a process which permits of this product being obtained abundantly and cheaply. It consists in submitting to the action of heat the hydrochlorate of trimethylamine, which is obtained as a by-product in the manufacture of potash of beets. The hydrochlorate is thus decomposed into free trimethylamine, ammonia, and chloride of methyl. A washing with hydrochloric acid takes away all traces of alkali, and the gas, which is gathered under a receiver full of water, may afterward be dried by means of sulphuric acid, and be liquefied by pressure.

VINCENTS ICE MACHINE. FIG. 2.--THE FREEZER (Transverse Section).
Pure liquid chloride of methyl is now an abundant product. There are two uses to which it is applied: first, for producing cold, and second, for manufacturing coal tar colors.
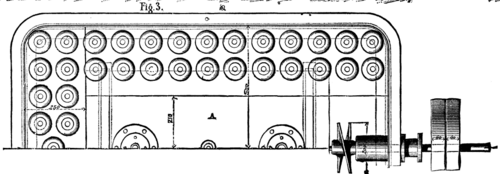
VINCENTS ICE MACHINE. FIG. 3.--HALF PLAN OF FREEZER
At present we shall occupy ourselves with the first of such applications--the production of cold.
The apparatus serving for the production of cold by this material are three in number: (1) the freezer (Figs. 1, 2, and 3), in which is produced the lowering of temperature that converts into ice the water placed in carafes or any other receptacles; (2) the pump (Figs. 4, 5, and 6), which sucks the chloride of methyl in a gaseous state up into the freezer and forces it into the liquefier; and (3) the liquefier, which is nothing else than a spiral condenser in which the chloride of methyl condenses, and from thence returns to the freezer to serve anew for the production of cold.
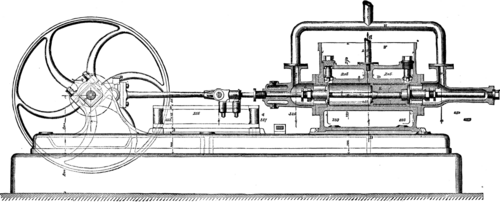
VINCENTS ICE MACHINE. FIG. 4.--THE PUMP (Longitudinal Section).
Continue to:


