Chapter I. Introduction
Description
This section is from the book "Distillation Principles And Processes", by Sydney Young. Also available from Amazon: Distillation Principles And Processes.
Chapter I. Introduction
Object Of Distillation
The object of distillation is the separation of a volatile liquid from a non-volatile substance or, more frequently, the separation of two or more liquids of different volatility.
If only one component of a mixture is volatile, there is no difficulty in obtaining it in a pure state by distillation, and in many cases the constituents of a mixture of two or more volatile liquids may be separated - though frequently at much cost of time and material - by means of the simple apparatus described in this chapter. For the fractional distillation in the laboratory of such complex mixtures as petroleum or fusel oil, the improved still-heads described in Chapters X. to XII. must be employed. Still-heads employed on the large scale are described in the sections of the book relating to alcohol, petroleum, etc.
Quantitative Analysis By Distillation
The determination, by ordinary analytical methods, of the relative quantities of two or more organic compounds in a mixture is often a matter of great difficulty, but, in many cases, the composition of the mixture may be ascertained approximately and, not seldom, with considerable accuracy from the results of a single distillation, if a very efficient still-head be employed. This method has proved of considerable value.
Difficulties Encountered
The subject of fractional distillation is full of interest owing to the fact that difficulties so frequently occur, not only in the experimental work, but also in interpreting the results obtained.
In the distillation of petroleum, with the object of separating pure substances, such difficulties are of common occurrence and are due to one or other of three causes: - (a) to the presence of two substances, the boiling points of which are very close together; (b) to the presence of one or more components in relatively very small quantity; (c) to the formation of mixtures of constant boiling point.
The separation of two liquids which boil at temperatures even 20° or 30° apart, such as ethyl alcohol and water, or benzene and isobutyl alcohol, may be impossible owing to the formation of a mixture of minimum or, less frequently, of maximum boiling point. It is, indeed, only in the case of substances which are chemically closely related to cach other that the statement can be definitely made that the difficulty of separating the components of a mixture diminishes as the difference between their boiling points increases.
In any other case, we must consider the relation between the boiling points, or the vapour pressures, of mixtures of the substances and their composition, and unless something is known of the form of the curve representing one or other of these relations, it is impossible to predict whether the separation will be an easy one or, indeed, whether it will be possible.
The form of these curves depends largely on the chemical relationship of the components, and it is now possible, in a moderate number of cases, to form an estimate, from the chemical constitution of the substances, of the extent to which the curves would deviate from the normal form, and therefore to predict the behaviour of a mixture on distillation.
Fractional distillation is frequently a very tedious process and there is necessarily considerable loss of material by evaporation and by repeated transference from the receivers to the still, but a great amount of both time and material may be saved by the use of a very efficient still-head ; and when the object of the distillation is to ascertain the composition of a mixture, very much greater accuracy is thereby . attained.
Apparatus
Ancient Apparatus
The process of distillation is evidently a very ancient one, for Aristotle mentions that pure water may be obtained from sea-water by evaporation, but he does not explain how the condensation of the vapour can be effected. A primitive method of condensation is described by Dioscorides and by Pliny, who state that an oil may be obtained by heating rosin in a vessel, in the upper part of which is placed some wool. The oil condenses in the wool and can be squeezed out of it.
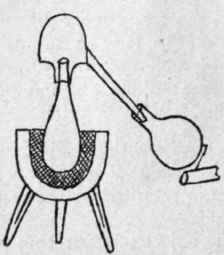
Fig. 1. - Alexandrian still with head, or alembic.
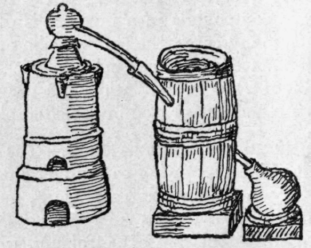
Fig. 2. - Ancient still with water condenser.
The Alexandrian chemists added a second vessel, the head or cover, called by the Arabians the alembic, to the boiler or still, and a simple form of apparatus used by them is shown in Fig. 1.
1 Kopp, Geschichte der Chemie, Beitrage i. 217.
Later on, the side tube was cooled by passing it through a vessel containing water. The diagram, Fig. 2, is taken from Libavius,
Syntagma Alchymiae Arcanorum, 1611.
Modern Apparatus
The apparatus employed at the present time is similar in principle, but, in addition, a thermometer is used to register the temperature.
In Fig. 3 the ordinary form of apparatus is shown, and we may distinguish the following parts : - The still, a ; the still-head, b; the Liebig's condenser, c, in which the vapour is deprived of heat by a current of cold water ; the receiver, d ; the thermometer, e. In the laboratory the still is usually heated by means of a Bunsen burner.
The flask or still is fitted with a cork through which passes the still-head, and the side delivery tube from the still-head passes through a second cork in the condensing tube. For liquids which boil at a high temperature, or which act chemically on cork, it is more convenient to have the still and still-head in one piece and to elongate the delivery tube so that it may pass, if necessary, through the Liebig's condenser (Fig. 4).
Continue to:


