The Testing Of Acetone And n-Butyl Alcohol. Part 3
Description
This section is from the book "Distillation Principles And Processes", by Sydney Young. Also available from Amazon: Distillation Principles And Processes.
The Testing Of Acetone And n-Butyl Alcohol. Part 3
Mixture Of Acetone And N-Butyl Alcohol
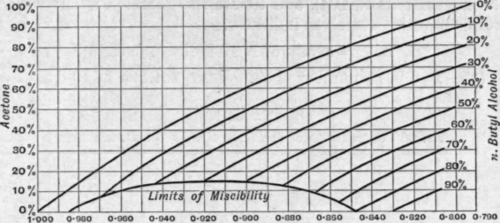
The amount of water in acetone or in n-butyl alcohol may be obtained from density curves when no other substance is present. These curves (see Figs. 99 and 100)1have been prepared from data given in Tables 98 and 99. From
1 Weizmann and Legg, Can. Pat., 202135/1920.
2Reilly and Hickinbottom, Trans. Chem. Soc, 1918, 113, 99; 1920, 117, 103.
3Briihl, Annalen, 1880, 203, 16. In the literature there appears to be some confusion between n and iso butyl alcohol, and it is probable that many of the recorded values in the literature for nutyl alcohol were not obtained with the pure substance.
4Ref. from Lecat. As a similar value is given for iso-butyl alcohol the value given is probably too high.
5Orton and Jones, Trans, Chem. Soc, 1919, 116, 1194.
Densities at 20/4o Fig. 99.
Fig. 99 the density of all possible mixtures of acetone, nbutyl alcohol, and water may be ascertained.
Table 98. Table of Densities and Contractions of Mixtures of Acetone and Water
Acetone per cent. | 20° D 20° found. | D20°/ 4o found. | D20°/ 4o calc. | Vol. 1 gram found. | Vol. 1 gram calc. | Contractions per cent. |
100 | 0.79231 | 0.79091 | 0.79091 | 1.26437 | 1.26437 | |
94.98 | 0.80832 | 0.80689 | 0.79923 | 1.2393 | 1.2512 | 0.951 |
89.58 | 0.82367 | 0.82221 | 0.80841 | 1.2162 | 1.2370 | 1.681 |
79.92 | 0.85171 | 0.85020 | 0.82535 | 1.1762 | 1.2116 | 2.922 |
71.10 | 0.87370 | 0.87215 | 0.84154 | 1.1466 | 1.1883 | 3.509 |
57.46 | 0.90629 | 0.90469 | 0.86753 | 1.1054 | 1.1527 | 4.103 |
50.03 | 0.92220 | 0.92057 | 0.88250 | 1.0863 | 1.13315 | 4.134 |
37.49 | 0.94667 | 0.94499 | 0.90893 | 1.0581 | 1.1002 | 3.827 |
29.62 | 0.95949 | 0.95779 | 0.92635 | 1.0441 | 1.0795 | 3.279 |
19.31 | 0.97480 | 0.97307 | 0.95012 | 1.0277 | 1.0525 | 2.356 |
9.93 | 0.98707 | 0.98532 | 0.97290 | 1.0149 | 1.02785 | 1.260 |
The methods (other than by density determination) available for the estimation of the water are limited to the use of a reagent which reacts or combines with the water alone without action on the other two constituents. Such a reagent as calcium carbide or an anhydrous salt might be used. A much simpler and more accurate procedure is to estimate the acetone by one of the methods available after suitably diluting with water. Of these, a modification of the method suggested by Messinger 2 or by Denige 3 is convenient, and gives good results.
Details of the method adopted are as follows: Dilute the sample with distilled water so that 10 c.c. diluted solution contains not more than 0.005 gram acetone. Take 10 c.c. of the dilute solution, add 5 c.c. 20 per cent soda solution, and run in from a burette
1 Reilly and Ralph, loc. cit. 2 Ber. 1888, 21, 33G6.
3 Comp. rend., 1898, 127, 963.
25 c.c. N/10 iodine solution. Shake at intervals for 15 minutes, keeping temperature at 15° C. Then add 5 c.c. 20 per cent Hc1 (of same strength as.the 20 per cent NaOH), and titrate the liberated iodine with N/10 thiosulphate, using starch as indicator.
Let x = excess of N/10 iodine in c.c.
Then (25-x) 0.967 = number of milligrams acetone in 10 c.c. of dilute sample.
Table 99
Densities and Contractions of Mixtures of n-Butyl Alcohol and Water.
n-Butyl Alcohol per cent. | D 20o 20o found | D 20o 4o found | D 20o 4o found | Vol. of 1 gram found. | Vol. of 1 gram calc. | Contraction per cent. |
100 | 0.81097 | 0.80953 | 0.80953 | 1.2353 | 1.2353 | • • • |
98.93 | 0.81318 | 0.81174 | • • • | • • • | • • • | • • • |
97.89 | 0.81538 | 0.81394 | 0.81277 | 1 .2286 | 1.2304 | 0.143 |
96.96 | 0.81731 | 0.81586 | • • • | • • • | • • • | • • • |
95.97 | 0.81935 | 0.81790 | • • • | • • • | • • • | • • • |
95.06 | 0.82108 | 0.81962 | 0.81716 | 1.2201 | 1.2238 | 0.302 |
93.98 | 0.82234 | 0.82088 | • • • | • • • | • • • | • • • |
93.02 | 0.82513 | 0.82367 | 0.82036 | 1.2141 | 1.2190 | 0.402 |
91.97 | 0.82689 | 0.82543 | • • • | • • • | • • • | • • • |
90.96 | 0.82883 | 0.82736 | • • • | • • • | • • • | • • • |
89.96 | 0.83066 | 0.82919 | 0.82522 | 1.2060 | 1.2118 | 0.479 |
89.26 | 0.83216 | 0.83068 | • • • | • • • | • • • | • • • |
88.06 | 083436 | 0.83288 | • • • | • • • | • • • | • • • |
83.03 | 0.84345 | 0.84196 | 0.83633 | 1.1877 | 1.1957 | 0.666 |
80.64 | 0.84777 | 0.84627 | • • • | • • • | • • • | • • • |
79.94 | 0.84917 | 0.84770 | 0.84140 | 1.1797 | 1.1885 | 0.740 |
7.90 | 0.98862 | 0.98687 | 0.98018 | 1.0133 | 1.0202 | 0.676 |
7.32 | 0.98946 | 0.98771 | 0.98164 | 1.0124 | 1.0188 | 0.628 |
7.06 | 0.98968 | 0.98793 | • • • | • • • | • • • | • • • |
611 | 0.99111 | 0.98936 | 0.98421 | 1.0108 | 1.0160 | 0.511 |
5.05 | 0.99244 | 0.99068 | • • • | • • • | • • • | • • • |
3.95 | 0.99382 , | 0.99202 | 0.98913 | 1.00805 | 10110 | 0.291 |
3.05 | 0.99532 | 0.99356 | • • • | • • • | • • • | • • • |
2.27 | 0.99651 | 0.99474 | • • • | • • • | • • • | • • • |
2.00 | 0-99678 | 0.99502 | 0.99364 | 1.0050 | 1.0064 | 0.139 |
1.61 | 0.99742 | 0.99566 | • • • | • • • | • . • | • • • |
1.04 | 0.99830 | 0.99653 | • • • | • • • | • • • | • • • |
0.61 | 0.99888 | 0.99711 | 0.99681 | 1.0029 | 1.0032 | 0.030 |
The estimation of the n-butyl alcohol requires a longer time, and may be carried out by a modification of the method of Verley and Bolsing for hydroxyl estimation.1
To a known weight of the "n-butyl alcohol, acetone, and water ' mixture anhydrous sodium sulphate was added in proportion to the water present (approximately estimated from the density), and the mixture extracted several times with xylene. The hydrocarbon extract was made up to a known volume. A measured amount was heated gently on a sand-bath, with an excess of a pyridine solution of acetic anhydride, contained in a large flask fitted with a reflux condenser. Two hours' heating is usually sufficient to complete the esterification.
1 Ber., 1901, 34, 3354.
Continue to:


