Dinitrochlorbenzene From Chlorbenzene
Description
This section is from the book "The Fundamental Processes Of Dye Chemistry", by Dr. Hans Eduard Fierz-David. Also available from Amazon: The Fundamental Processes of Dye Chemistry.
Dinitrochlorbenzene From Chlorbenzene
The nitration of chlorbenzene takes place very readily; it is nitrated first only to the mono-nitro stage, as given under the preparation of dinitrobenzene. The product is a mixture of ortho- and para-derivatives which is not easy to separate as their boiling-points are very close to one another, namely, 243 ° and 2420 for the ortho- and para- compounds respectively.
For this reason the separation is always effected by freezing out, centrifuging and distilling in vacuo with a very tall column. This operation cannot be properly carried out in the laboratory, but it is quite easy to separate a large portion of the para product in a pure condition from the crude mixture of the two nitrochlorbenzenes by freezing out and pressing. The nitrochlorbenzenes are very poisonous, for which reason the centrifuges in which the product is "whizzed" must be very carefully built, provided with well-fitting covers with flues similar to those in which, for instance, gun-cotton is dehydrated by means of alcohol.
We will therefore not discuss the preparation of the mono-nitro compounds, but will proceed directly with the further nitration of the mixture.
350 Gms. of mixed acid containing 50 % Hno3 are placed in an iron nitrating vessel (Fig. 2), and into this is dropped 113 gms. chlorbenzene with good stirring, keeping the temperature below 50. After all has been added the stirring is continued for a further hour at 5-10°. The temperature is then raised slowly to 500, and kept for a further hour at this temperature. 350 Gms. concentrated sulphuric acid are then dropped in very cautiously with continued vigorous stirring, the mixture being finally heated for half an hour at 1150. After cooling, the nitrated product is poured into 2 litres of water, in which it immediately solidifies to a pale yellow cake. This is separated from the mother-liquor, melted under water to remove all acid, and is then chemically pure.1
The yield is 200 gms. from 113 gms. chlorbenzene. M.p. 51o.
Notes on Works Technique and Practice. - The manufacture of dinitrochlorbenzene has assumed quite unexpected proportions. It serves for the preparation of Sulphur Black T (q.v.) and other important dyes. Further, it is the starting-point for a whole series of condensation products which are obtained by replacing the mobile
350 gms. mixed acid (50 % Hno3).
113 gms.
Chlorbenzene.
350 gms. conc. H2S04 (66° Be.).
1 It is advisable to offer a word of warning as to the extremely unpleasant properties of dinitrochlorbenzene. Both as a solid and, more especially, in its solutions it produces eczema and unbearable itching.
chlorine atom by basic and other radicals. Thus, for instance, hexanitrodiphenylamine is obtained from it, which is one of the most powerful of present-day explosives (torpedoes).1 Further, dinitraniline, dinitrophenol, and picric acid can be readily obtained from it. The diagram given below shows only a small portion of the various practical possibilities.
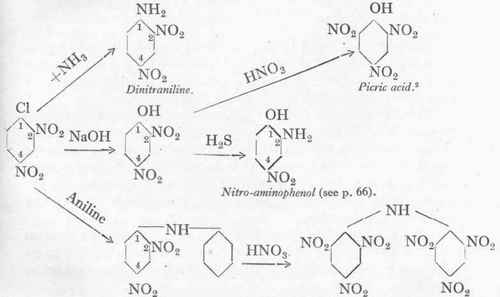
Dinitrodiphenylamine.
Hexanitrodiphenylamine.
We have seen that in the laboratory a 30 % excess of nitric acid is used in order to obtain smoother nitration. On the large scale it is possible to manage with a much smaller excess, and even this is finally recovered by separating the waste acid into sulphuric and nitric acids in denitrating towers by means of steam. Dinitro-chlorbenzene costs less than 90 centimes per kilo.
Continue to:


