Special Directions - Gold. Part 8
Description
This section is from the book "Leaching Gold and Silver Ores. The Plattner And Kiss Processes: A Practical Treatise", by Charles Howard Aaron. Also available from Amazon: Leaching Gold And Silver Ores.
Special Directions - Gold. Part 8
Calcium Hyposulphite
150. Calcium Hyposulphite. This solvent may be made by passing air, and the fumes from burning sulphur, or from sulphuric acid and charcoal heated in a retort, through a solution of calcium polysulphide until the latter is colorless. It is better to buy a barrel or two of sodium hyposulphite in crystals, with which to make the leaching solution to begin with. In use, with calcium sulphide as the precipitant, the sodium hypo soon disappears, being replaced by calcium hypo through the chemical reactions which take place.
151. The strength of the solution to be used for leaching depends somewhat on the composition of the ore. If this contains but little base metal, the solution may be quite strong, and is even used warm. But, in general, a strong solution would extract too much base metal. It may be made by dissolving two pounds of crystallized sodium hyposulphite in each cubic foot of water, or about 26 1/2 pounds to 100 gallons. If it is then found to dissolve too much base metal, which may be ascertained by an examination, or an assay, of some of the precipitate, the strength is reduced by an addition of water. After it has been used the density cannot be relied on, as it then contains other substances besides hyposulphite. A good guide is the taste, which should be very sweet during the first stage of the leaching, if the ore contains much silver. The solvent power of the solution may at any time be tested, thus: Dissolve 5.25 grains of pure silver in nitric acid; precipitate as chloride, by adding a little hydrochloric acid. Wash the precipitate three or four times with water, to remove every trace of acid. One fluid-ounce of the leaching solution should dissolve the whole of the silver chloride. Some operators, when in want of leaching solution for silver ores, obtain it by treating with calcium polysulphide the solution of base metal chlorides resulting from the preliminary washing of the roasted ore. The metals are thrown down as sulphides, and the solution then contains calcium chloride, together with the calcium hyposulphite previously existing in the precipitant (148).
Working Test
152. Working Test. The apparatus used is represented in Figure 1, Plate 6. The generator and wash-bottle are made of two wide-necked bottles, jars, or flasks, with corks, and some glass tubes. The corks should be soaked in melted paraffin, and the S tube is enlarged at the upper end, so as to form a small funnel, into which to pour the acid. If preferred, a glass generator and two-necked wash-bottle can be bought of J. Caire, in San Francisco.
A chlorinating vat is made of a common wooden pail, or small tub, in the bottom of which, near the side, a hole is bored and. a cork inserted. Through the cork is passed a piece of 1/4-inch glass tube 4 inches long. The cork and tube must not project above the bottom of the pail inside. A wooden cover is made to fit into the pail half an inch below the rim, and in it is a hole, fitted with a cork and tube similar to those in the bottom. The pail, while dry, is thoroughly coated inside with melted paraffin, which is caused to soak into the wood a little by the aid of heat. A filter is made in the vat by means of a layer of pebbles, covered with a piece of moistened grain sack, or similar material.
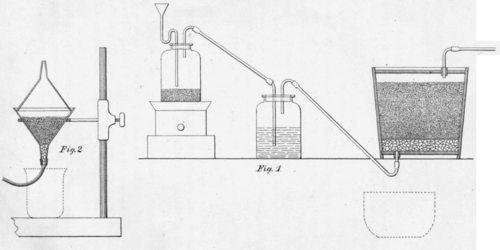
Plate VI.
A weighed quantity, from 10 to 20 pounds of the pulverized ore, or concentrations, is dead roasted or chloridized, in a small reverberatory furnace, with the precautions indicated, according to the character of the ore. When cooled, it is slightly moistened, and thrown on the filter in the vat. A space of not less than half an inch must be left between the surface of the ore and the cover, which is now placed, and luted with dough, or with a paste made from a mixture of equal parts of flour and paris plaster, which will not crack in drying.
Chlorine is generated from manganese and hydrochloric acid, the latter being more convenient for small operations than the sulphuric acid and salt used on the large scale. Three ounces of manganese are put into the generator, moistened with water, and warmed on a sand bath resting on a small coa!-oil stove. The acid is gradually added by means of the S tube. The exit tube of the generator is connected with the wash-bottle, and that with the glass tube in the bottom of the vat, by rubber tubing.
When a glass rod, dipped in ammonia and held to the tube in the cover of the vat, causes the formation of dense fumes, indicating the escape of chlorine, the surplus gas is conveyed out of the room by a rubber tube connected with the tube in the cover.
The chlorine is allowed to pass through the ore for an hour, more acid being poured into the generator when required, to maintain the evolution of gas. The waste pipe is then closed by a pinchcock. The wash-bottle is disconnected from the generator, but not from the vat, unless it is required for another operation, in which case the glass tube in the bottom of the vat is effectually, and conveniently, closed by immersing its lower end in melted stearin, paraffin, or tallow, contained in a small cup (dry cup), which is then allowed to congeal.
After the lapse of from 20 to 40 hours, as may be required, the cover is removed. A rubber tube, connected with the glass tube in the bottom of the vat, is arranged to deliver into a glass, or porcelain vessel, capable of containing about a gallon, and is closed by a pinchcock.
Water is now sprinkled on the ore, and when the latter has settled, more water is poured in until it is covered. After half an hour, the pinchcock on the discharge pipe is adjusted so as to allow the leach to flow in a slow stream, water being poured upon the ore from time to time to keep it covered. The leaching is continued until a sample, received in a test tube, no longer gives the slightest precipitate on addition of solution of iron sulphate. A more delicate test is that with tin protochloride, which, with the slightest trace of gold, gives a purple coloration.
The gold is precipitated by adding to the leach a strong solution of iron sulphate, which is thoroughly mixed by stirring with a glass rod. To ascertain if enough of the iron sulphate to precipitate the whole of the gold has been used, a drop of the liquid is transferred, by means of the glass rod, to a porcelain dish, or a saucer, and is brought into contact with a drop of solution of potassium ferridcyanide, or "red prussiate of potash." If an intense blue coloration is not produced, more iron sulphate is required.
The gold requires 12 hours to settle, after which the greater part of the clear liquid may be removed by means of one of the rubber tubes, applied as a siphon. The remainder, with the gold, is thrown on a paper filter, or the whole of the liquid may be passed through the filter, to insure the collection of every particle of the metal.
The sides, and bottom of the vessel in which the precipitation was effected, and the glass rod with which the stirring was performed, are carefully wiped with pieces of filter paper, held in the forceps, to remove adhering gold, and the paper is added to the gold on the filter. The filter, with the metal, is dried in the funnel, and is then placed in an assay crucible, together with an ounce or more of litharge, and a little borax, and smelted. The filter reduces a sufficient quantity of litharge to metallic lead, for the collection of the gold, which is then separated by cupellation, weighed, and the result compared with the assay of the ore.
If the ore contains silver, the gold on the filter may be washed with water, then, with the entire filter, drenched with ammonia, and again washed, before being dried. This will remove any silver chloride which may be present, or the bead may be inquartated and parted in the usual way. After the extraction of the gold, the ore, which in this case should have been roasted with salt, is leached with hypo for silver as long as a precipitate is produced by the addition of a drop of calcium sulphide to a sample of the leach.
The silver is then precipitated with calcium sulphide, as in the large way, except that, as it is not necessary to preserve the hypo, an excess of the sulphide is used, so that not a trace of the metal may be lost. The precipitate is coagulated by heating on a sandbath, separated from the liquid by filtration, dried, and dressed on the filter with litharge and borax, then fused in a crucible, with the addition of a little nitre to prevent the production of too much lead by the action of the sulphur on litharge. The lead button obtained is cupelled, and the resulting silver bead, after weighing, should be subjected to parting, as it may contain a little gold.
The tailings are dried and weighed. The loss of weight found to have been sustained by the ore in the working is reduced to percentage. An assay of the tailings is then made, and from the result the same percentage is deducted. The remainder is the loss per ton of ore by insolubility. This is added to the amount per ton extracted, and the sum deducted from the assay value of the original ore. The remainder is the loss per ton by volatilization, dusting, and other causes.
153. A smaller test may be made in the laboratory. Half an ounce, or an ounce, of ore, is roasted in the muffle, with the precautions indicated under "Assaying Concentrations" (160), with or without salt, as required, and is chlorinated in the apparatus represented in Fig. 2, Plate 6, formed of two glass funnels. In the neck of the lower funnel some fragments of broken glass are placed, and on them a filter is constructed of rather coarsely powdered glass. Upon the filter the moistened ore is placed, the upper funnel is then arranged as a cover, and luted with a paste of flour and paris plaster. Two ore sample bottles, fitted with corks and tubes, as in Figure I, suffice for a generator and wash bottle. The corks should be saturated with paraffin or tallow.
This operation has the advantage that, after the precious metal has been extracted and collected, as in the larger test, the whole of the ore and glass filter, dried in the funnel, can be dressed as an assay and smelted, so that it not only shows what can be extracted, but it also gives the absolute loss by insolubility, and, by difference, the loss in roasting, without complications arising from a change of weight in working.
154. Change in Weight. When working on the large scale by lixiviation, it must be remembered that the ore not only gains or loses weight in the roasting, but also loses in the leaching by the amount of soluble matter, of whatever kind, extracted. The tailings assay, therefore, does not represent the real loss by insolubility unless corrected. The change of weight sustained in roasting and leaching, is approximatively obtained by weighing, roasting, leaching, drying, and reweighing a number of small samples, using the same proportion of salt, and, as nearly as possible, the same heat in roasting, as in the large way. The loss of weight thus found, when reduced to percentage, gives the correction to be applied to the assay value of the tailings. Thus, if tailings assay 2.6 oz. per ton, and the loss of weight in roasting and leaching has been found to average 22 per cent, the real loss per ton of crude ore, from insolubility, is 2.6 - 0.57=2.03 oz.
Continue to:


